Abstract
Background The classification of PCDLBCL and the exact distinction between NOS and LT are still a matter of debate. To contribute to this discussion we analyzed the complete clinico-pathological, cytogenetic and molecular features of a series of PCDLBCL and, for comparison, of DLBCL secondarily localized to the skin, diagnosed and treated in 4 Italian centers. Here, we highlight biologic similarities and differences between primary and secondary cases, particularly focusing on the cytogenetic and molecular features.
Patients and Method
We analyzed clinical data and skin biopsies of pts diagnosed as having PCDLBCL when they had no previous history of DLBCL and other lesions were excluded by clinical and radiological examination. All other pts were considered as having SCDLBCL. During a session at a multi-head microscope, the pathologists reviewed all slides. By Hans algorithm, cases were classified as GC and non-GC. Based on the histopathological and IHC features and clinical data, PCDLBCL were segregated into PCDLBCL-LT and PCDLBCL-NOS (Lucioni M., Cancer Med, 2016). FISH analysis was performed and interpreted at the Laboratory of cytogenetic of Varese University hospital as previously described (Tibiletti M.G., Cancer Genet Cytogenet, 2004). On DNA and RNA from FFPE, we performed the analysis of MYD88 mutations by NGS and COO classification by the Lymph2Cx assay that subdivides DLBCL into ABC, GCB and Unclassified subsets.
Results
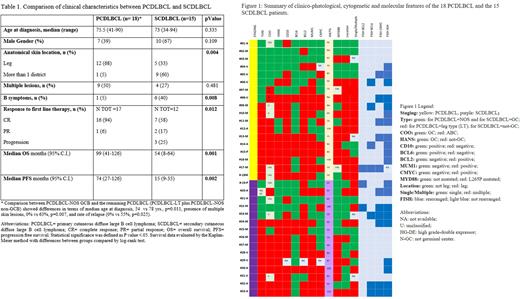
Clinical characteristics of the 33 CDLBCL pts are summarized in Tab 1. Figure 1 summarizes the clinical data and pathological, immunophenotypic, cytogenetic and molecular results of 18 PCDLBCL and 15 SCDLBCL pts. PCDLBCL were classified as LT and NOS in 56% and 44%, respectively. BCL2, MYC and DE phenotype were less expressed in NOS pts,50%, 29% and 14%, respectively than in LT cases ,100%, 50% and 50%, respectively. The results of the interesting comparison between PCDLBCL-NOS GCB with the remaining PCLBCL are reported in Tab1. Lymph2Cx subdivided the 28 CDLBCL in 17 ABC, 7 GCB and 4 Unclassified subtypes. Among PCDLBCL-LT pts, we did not find any GCB case, while it represented the most frequent type among PCDLBCL-NOS pts. Classification by COO and Hans algorithm was concordant in 64 % of pts. FISH analysis showed that 23 of the 33 pts had at least one gene rearrangement, including 13/18 pts with PCDLBCL and 10/15 pts with SCDLBCL. All cases had IGH rearrangement. MYC rearrangement was found in 5 pts with PCDLBCL and in 6 with SCDLBCL. BLC6 was rearranged in 4 pts with PCDLBCL and in 3 with SCDLBCL, while BCL2 was rearranged in 1 patient with PCDLBCL and in 2 with SCDLBCL. Finally, we observed that 8/9 pts with PCDLBCL-LT had at least one driver gene rearranged, while only 5/9 PCDLBCL-NOS were rearranged.MYD88 mutations were more frequent in PCDLBCL- LT than in PCDLBCL-NOS. Among SCDLBCL cases, MYD88 mutation was found in 47% of pts with the non-GC subtype. Significantly, none of the GC pts resulted positive. MYD88 mutated pts were older and with a non-GCB phenotype, compared to MYD88 WT pts. MYD88 WT CDLBCL pts had a significant better OS compared to the pts with L265P mutation. Among the PCDLBCL pts, in terms of both OS and PFS the most relevant clinical prognostic factors were age and MYD88 mutation. Among the SCDLBCL, only relapse and high expression of Ki67 are associated with shorter OS (Tab1).
Discussion
Our study emphasizes the clinico-pathological, cytogenetic and molecular similarities and differences between PCDLBCL and SCDLBCL, underlying the importance of properly staging CDLBCL pts at time of diagnosis . In the PCDLBCL setting, we confirmed the independent pathological entity of the NOS category highlighting the better prognostic outcome of this subtype.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal